Pfizer and German company BioNTech SE on Monday announced a vaccine candidate for COVID-19, which the world has been battling since early this year. The companies said BNT162b2 has proved successful in the first interim analysis from the phase three study.
Pfizer began phase three of the clinical trial on July 27, with 43,538 participants enrolled. Participants in the “blinded” trial receive either the vaccine candidate or a saltwater placebo, meaning no one except the independent board knows which one the patients received, The New York Times reported.
Of the 43,538 participants, 94 displayed symptoms of coronavirus indicating that the vaccine’s efficacy rate is 90 percent, which represents the percentage of participants who received the vaccine and did not display symptoms.
A total of 38,955 participants have received the second dose as of November 8.
Although the vaccine appears promising, the trial must continue until 164 coronavirus cases have accrued, and there are still unknowns.
Efficacy vs. Effectiveness
Pfizer’s report of an “efficacy rate above 90%” has led many to believe that the vaccine is 90 percent effective. Yet there is a difference between the two.
Zania Stamataki, a viral immunologist at the University of Birmingham, clarifies the difference in a piece with The Conversation. “…Efficacy is the performance of a treatment under ideal and controlled circumstances, and effectiveness is performance under real-world conditions,” Stamataki said.
What does this mean for the trial? Clinical trials take place in a controlled environment to test if the vaccine is safe and if it works. The 90 percent efficacy rate means that COVID-19 symptoms were prevented for 90 percent of participants who received the vaccine, though Stamataki predicts that the number will change by the end of the trial.
Vaccines with a 50 percent efficacy rate are expected to be approved for COVID-19, Stamataki said.
Is the Vaccine Safe?
The Data Monitoring Committee from the phase three trial analysis has not reported “any serious safety concerns.”
Pfizer and BioNTech still are collecting safety data, and Stamataki expects that the safety data needed for the FDA’s possible Emergency Use Authorization, a median of two months of safety data collecting following the second (and final) dose of vaccine, will be released during the third week of November.
Additionally, trial participants will continue to be monitored for “long-term protection and safety” for two years after their final dose, even after the vaccine is made available to the public.
What Does This Mean for the Public?
“Pfizer’s chief executive has said that it could have 30 to 40 million doses of the vaccine before the end of the year, enough for 15 to 20 million people to get an initial shot and a booster three weeks later,” The New York Times reported. It is unknown who will receive the vaccine first, but it is likely to be those of high-risk groups and frontline workers.
“Approximately 42% of global participants and 30% of U.S. participants have racially and ethnically diverse backgrounds,” according to Pfizer.
Pfizer and BioNTech expect to produce up to 1.3 billion doses of vaccine in 2021, yet that is far from the number needed to meet worldwide demand. If other companies prove to have successful vaccines, the demand could possibly be met. At this point, 10 other vaccines around the world also are in late-stage trials, according to The New York Times.
Until a vaccine is approved, and possibly after, health professionals and officials urge the public to remain socially distanced and wear a mask.
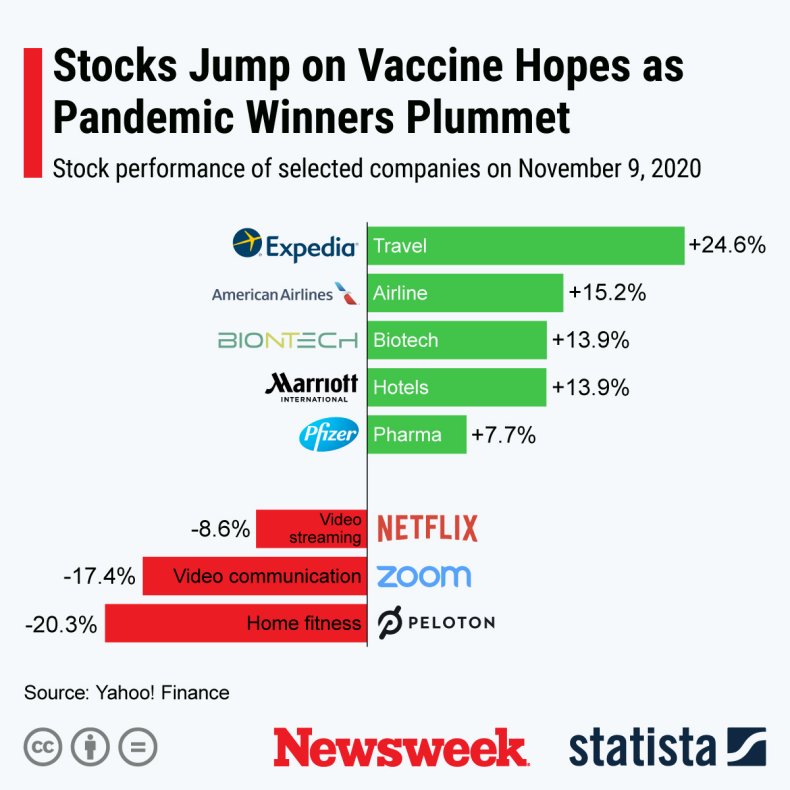 Stocks performance after the news of a promising coronavirus vaccine candidate. Statista
Stocks performance after the news of a promising coronavirus vaccine candidate. Statista


















