The United Kingdom on Wednesday became the first nation in the world to approve the COVID-19 vaccine developed by Pfizer/BioNTech, and elderly people in care homes and their staff will be the first to be inoculated, as early as next week.
The priority list for who will be vaccinated first was made at the suggestion of the Joint Committee on Vaccination and Immunisation (JCVI), a group of independent experts who advise the government on vaccine safety and use.
As of Wednesday, the committee’s advice is that the vaccine should be given to care home residents and staff, followed by people over 80 and front-line health workers, then the rest of the population in order of age and risk level.
The full list is as follows:
- Older adults in a care home and care home workers
- All those 80 and over and health and social care workers
- All those 75 and over
- All those 70 and over
- All those 65 and over
- High-risk adults under 65
- Moderate-risk adults under 65
- All those 60 and over
- All those 55 and over
- All those 50 and over
- Rest of the population (priority to be determined)
The U.K. said it has ordered 40 million vaccine doses from Pfizer/BioNTech, of which it expects 10 million to arrive by the end of the year. The country said it is planning to roll out the first phases of the vaccine program within the next week.
The vaccine requires two shots per person, so 40 million doses will be enough to vaccinate 20 million U.K. citizens, or roughly one-third of the population.
“The vaccine will be made available across the U.K. from next week. The NHS has decades of experience in delivering large-scale vaccination programs and will begin putting their extensive preparations into action to provide care and support to all those eligible for vaccination,” a spokesperson for the Department of Health and Social Care said, referring to the National Health Service.
Scottish First Minister Nicola Sturgeon said the first people in her country will be vaccinated on Tuesday, the BBC reported.
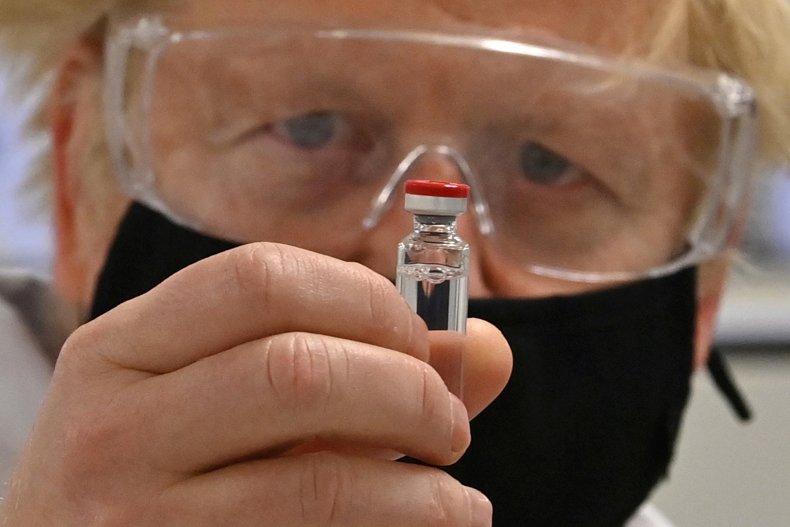
U.K. Prime Minister Boris Johnson poses for a photograph with a vial of a coronavirus vaccine at a pharmaceutical manufacturing facility in Wrexham, Wales, on November 30. The U.K. became the first nation to approve a vaccination program and will begin inoculating elderly adults in care homes and staff first. Paul Ellis – WPA Pool/Getty
The JCVI announced that the priority list is subject to change if more information becomes available about vaccine safety and effectiveness in different age groups. But the committee said the current age-based list will “likely result in faster delivery and better uptake in those at the highest risk.”
So far, there are no plans to make the vaccine mandatory throughout the U.K. The program will instead be administered and managed by health agencies in each country, including NHS England and NHS Improvement, NHS Wales, NHS Scotland, and Health and Social Care Northern Ireland.
The Pfizer/BioNTech vaccine is reportedly 95 percent effective and needs to be stored at a temperature of -70°C.
Temperature-controlled thermal shippers, which use dry ice to maintain the recommended conditions, can be used as temporary storage units for 15 days by refilling them with dry ice. When the vaccine is stored in a refrigerator at temperatures of 2-8 degrees C, it has an effective life of up to five days, which the government says will allow it to be easily distributed.
The BBC reported that roughly 50 hospitals are on standby to receive the vaccine, and additional immunization venues, such as conference centers and sports stadiums, are being set up now.
On Wednesday, U.K. Prime Minister Boris Johnson tweeted that news of the vaccine approval was “fantastic.”
“It’s the protection of vaccines that will ultimately allow us to reclaim our lives and get the economy moving again,” he wrote.